- Application Cases
- Hot Sales
- Products
- Application
- About Us
- Support
- News
- Contact
Views: 0 Author: Site Editor Publish Time: 2025-12-09 Origin: Site











Protein content is a core quality indicator for protein powder products, directly reflecting product value and nutritional efficacy. In food production and quality supervision, accurate determination of protein content not only complies with national food safety standards (such as GB 5009.5-2010) but also ensures that consumers get products that meet nutritional claims. Traditional protein testing methods often involve cumbersome manual operations, long digestion and titration cycles, and high reliance on operator experience—leading to problems such as inconsistent results and low efficiency. With the development of analytical technology, automatic potential titrators have become a game-changer in protein testing, solving the pain points of traditional methods with high precision, automation, and stability. The GT70 Automatic Potential Titrator, with its dual-channel design, corrosion-resistant structure, and intelligent operation system, provides a reliable and efficient solution for protein content determination in protein powder.Protein powder generally refers to a powdered product composed of purified soy protein, casein, whey protein (lacking isoleucine), pea protein, or a combination of the above proteins (such as a composite protein consisting of soy protein, whey protein, and pea protein). Protein is the most important nutrient for ensuring physical health; it is essential for maintaining and repairing the body and cell growth. It not only affects the growth of body tissues such as muscles but also participates in hormone production, immune function maintenance, transportation of other nutrients and oxygen, hemoglobin formation, blood coagulation, and many other aspects. However, excessive protein intake is not only a waste but also harmful to human health. Therefore, accurate determination of protein content in protein powder is of great significance for product quality control, compliance with nutritional labeling requirements, and protection of consumer health.

Proteins in food are decomposed under catalytic heating conditions, and the produced ammonia combines with sulfuric acid to form ammonium sulfate. Alkaline distillation frees the ammonia, which is then absorbed by boric acid and titrated with a standard sulfuric acid or hydrochloric acid titrant. The protein content is calculated by multiplying the acid consumption by a conversion factor. This method is in full compliance with the national standard GB 5009.5-2010, and the GT70 Automatic Potential Titrator enhances the accuracy of endpoint detection through real-time potential monitoring, avoiding the subjectivity of manual colorimetric endpoint judgment in traditional methods.
Sample pretreatment equipment: Graphite digestion furnace, Kjeldahl nitrogen analyzer (for sample digestion and distillation)
Core detection and analysis equipment: GT70 Automatic Potential Titrator (equipped with a 2-channel fluid-addition module, imported corrosion-resistant PTFE pipelines and rotary valves, supporting automatic sampling, cleaning, and data recording)
Auxiliary equipment: Analytical balance (accuracy to 0.1mg), 300ml digestion tubes, waste gas absorption system, etc.
The GT70 Automatic Potential Titrator stands out with its core advantages: 1) The 2-channel fluid-addition module enables parallel titration or rapid reagent switching, improving testing efficiency; 2) High-precision closed-loop control and 1μL burette resolution ensure a titration volume accuracy of 0.001mL, meeting the strict requirements of trace analysis; 3) The power-on self-check function and fully sealed pipeline design reduce equipment failure and cross-contamination risks; 4) Compliance with GMP, GLP, and FDA standards, with audit tracking and hierarchical user rights management, ensuring data integrity and traceability.
Titrant: C (H₂SO₄) = 0.100mol/L (calibrated in accordance with national standard GB/T 601-2002)
Boric acid solution: 20g/L (for absorbing distilled ammonia)
Indicator: Methyl red-bromocresol green ethanol mixed solution (volume ratio 1:5)
Catalyst tablet: Contains 0.2g copper sulfate and 3g potassium sulfate (promotes protein decomposition)
Sample: Commercially available protein powder
Other reagents: Concentrated sulfuric acid (analytical grade), distilled water, etc.
Weigh 0.2g of protein powder sample (accurate to 0.1mg) using an analytical balance, wrap it in dust-free weighing paper, and put it into a 300ml digestion tube to ensure no sample loss.
Add 1 catalyst tablet and 10ml concentrated sulfuric acid to the digestion tube, and shake gently to fully mix the sample with the reagents; perform a blank experiment at the same time (no sample added, other operations are the same). Place the digestion tube rack on the graphite furnace, cover the waste gas hood, and turn on the waste gas absorption system. Use a gradient temperature rise mode for digestion: maintain at 200℃ for 20min, then at 460℃ for 60min. After digestion is completed, take out the digestion tube and cool it to room temperature.
After the Kjeldahl nitrogen analyzer completes cleaning and blank distillation, set the instrument according to the following parameters: dilution water volume 10ml, boric acid volume 30ml, alkali addition volume 40ml, distillation time 5min, rinsing water volume 10ml. Install the cooled digested sample on the Kjeldahl nitrogen analyzer for distillation, and the ammonia is absorbed by the boric acid solution to form ammonium borate solution.
Transfer the absorbed solution to the titration cup of the GT70 Automatic Potential Titrator, select the "acid-base titration" mode and set it to "constant titration" on the instrument software. The instrument automatically uses 0.1mol/L sulfuric acid standard titrant for titration, real-time displays the pH-titration volume curve, and intelligently identifies the endpoint based on potential changes—eliminating manual operation errors. After the titration, the instrument directly calculates the test result according to the preset formula, and the data is automatically stored in the built-in 300G storage space (supporting up to 10,000 sets of data storage).
Blank volume: V0 = 0.41ml
Sulfuric acid titrant concentration: C = 0.104mol/L
Conversion factor of nitrogen to protein (for soy protein products): F = 6.25
The test data of 6 parallel samples are as follows:
| Serial Number | Sample Mass m(g) | Titration Volume V1(ml) | Nitrogen Content (%) | Protein Content (%) |
|---|---|---|---|---|
| 1 | 0.20770 | 19.303 | 13.25 | 82.84 |
| 2 | 0.19744 | 18.310 | 13.21 | 82.56 |
| 3 | 0.20416 | 18.961 | 13.24 | 82.75 |
| 4 | 0.20534 | 18.962 | 13.16 | 82.28 |
| 5 | 0.20166 | 18.820 | 13.31 | 83.19 |
| 6 | 0.20226 | 18.871 | 13.31 | 83.19 |
| - | Average Value | - | 13.25 | 82.80 |
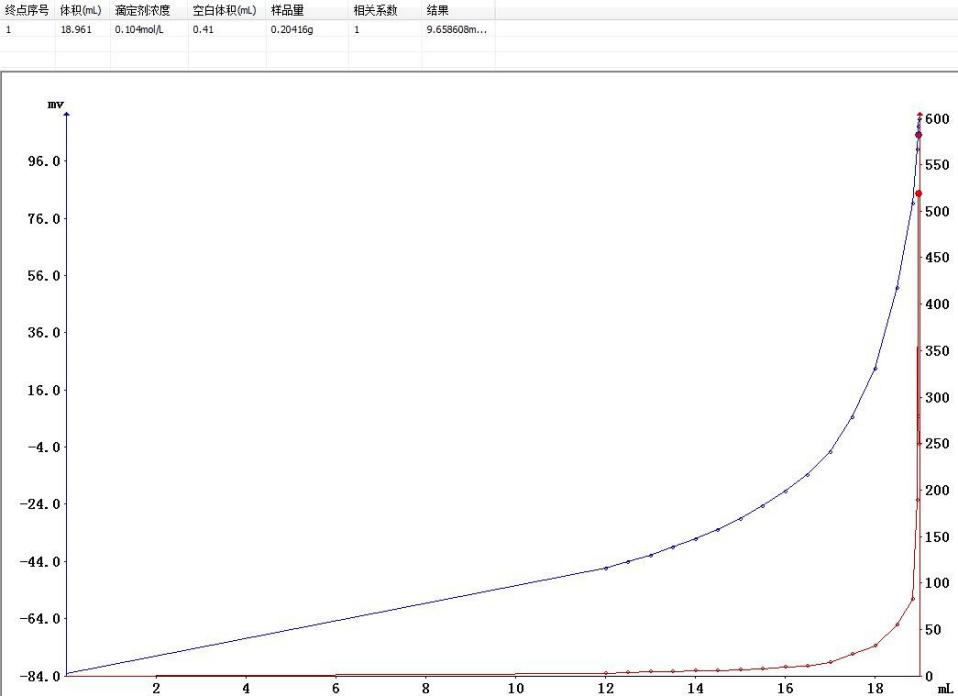
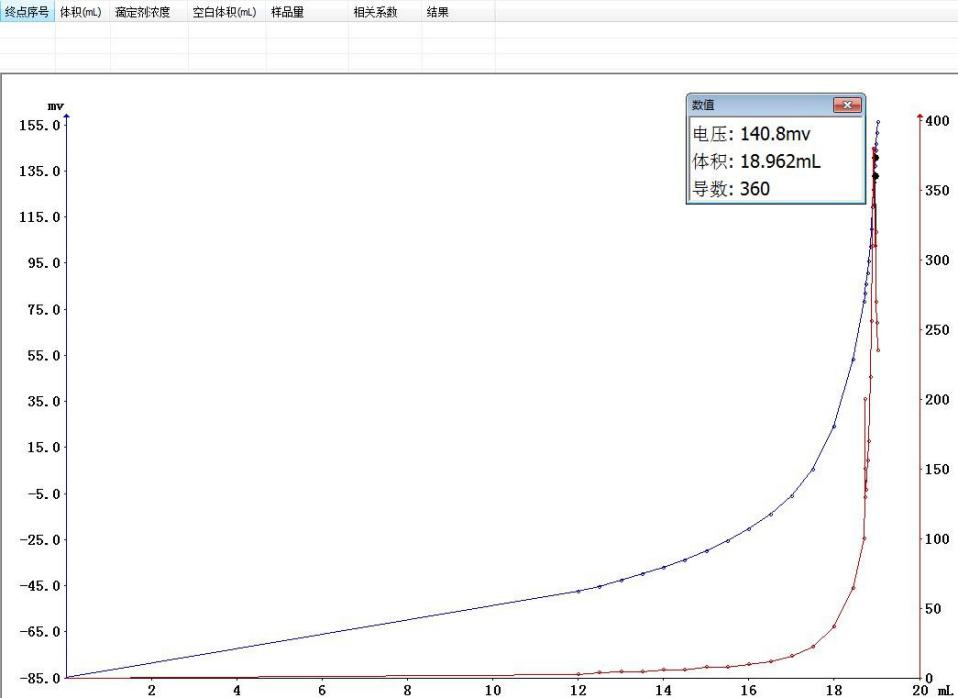
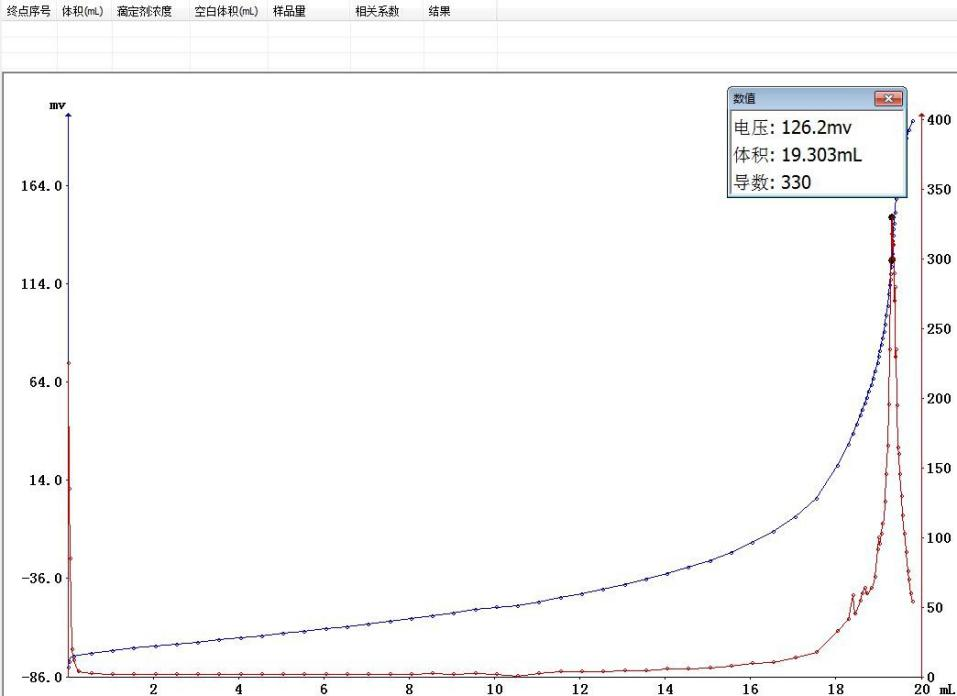
Nitrogen content calculation formula:W (%) = [1.401 × C × (V1 - V0)] / mWhere: W is the nitrogen content of the sample (%); C is the concentration of the sulfuric acid titrant (mol/L); V0 is the blank titrant consumption volume (ml); V1 is the sample titrant consumption volume (ml); m is the sample mass (g).
Protein content calculation formula:Protein Content (%) = W × FWhere: F is the conversion factor of nitrogen to protein (6.25 for soy protein products).
Three sets of typical sample titration curves were selected for analysis:
Sample mass 0.20416g: Endpoint volume 18.961ml, obvious potential mutation, high endpoint recognition accuracy;
Sample mass 0.20534g: Endpoint volume 18.962ml, good curve repeatability, reflecting instrument stability;
Sample mass 0.20770g: Endpoint volume 19.303ml, positively correlated with sample mass, consistent with titration theory.
The relative standard deviation (RSD) of the protein content of the 6 parallel samples is less than 0.5%, which meets the precision requirements of national standard methods. This verifies that the GT70 Automatic Potential Titrator has excellent repeatability, and its imported micro-detection electrode and high-power magnetic stirrer ensure uniform mixing of the solution during titration and accurate capture of potential changes.
Detected by the GT70 Automatic Potential Titrator, the average nitrogen content of the commercially available protein powder is 13.25%, and the average protein content is 82.80%. The test results are accurate and reproducible, fully meeting the precision requirements for protein content detection in the food industry. The entire experimental process (from sample pretreatment to result output) is efficient and fast, significantly shortening the detection cycle.
In protein powder protein content testing, the GT70 Automatic Potential Titrator demonstrates significant advantages such as high accuracy, strong automation, and compliance with standards. Compared with traditional manual titration, it not only reduces human errors caused by manual operation and endpoint judgment but also realizes automatic data recording, calculation, and storage—greatly improving testing efficiency and data integrity. Its corrosion-resistant PTFE pipeline and dual-channel design make it suitable for large-batch sample testing in food factories, quality inspection institutions, and research laboratories.Based on the Kjeldahl nitrogen method principle and combined with the precise titration capability of the GT70 Automatic Potential Titrator, this experiment successfully achieved efficient determination of protein content in protein powder. The instrument is easy to operate, stable in performance, and compliant with industry standards such as GMP and GLP, with traceable and auditable data, providing reliable technical support for protein detection in the food industry. Whether it is quality control in production enterprises or supervision and sampling inspection by regulatory authorities, the GT70 Automatic Potential Titrator is a trustworthy analytical tool.For more details about the GT70 Automatic Potential Titrator, please visit the official website.
GB/T 601-2002 Chemical Reagents - Preparation of Standard Titration Solutions[S]. Beijing: China Standards Press, 2003.
GB 5009.5-2010 National Food Safety Standard - Determination of Protein in Food[S]. Beijing: China Standards Press, 2010.